|
|
Cladoendesis
and a new
look at the evolution of insect metamorphosis
Kluge
N.J.
St. Petersburg State University, St. Petersburg, Russia
Entomological Review,
2012
volume 92, issue 6, pp. 622–632 
Translated
from:
Entomologicheskoe
Obozrenie 2012
91 (1): 63–78 
|
RUSSIAN
|
emblem of the conference,
drawn by the author of the paper; according to the principles of
cladoendesis, such insect can't exist
|
 |
|
 Introduction
Introduction
 1. Principles of cladoendesis
1. Principles of cladoendesis
 2. Conservation of characters
2. Conservation of characters
 3. Cladoendesis and the study of metamorphosis
3. Cladoendesis and the study of metamorphosis
 3.1. Amyocerata: the evolution of molt-related processes in antenna
3.1. Amyocerata: the evolution of molt-related processes in antenna
 3.2. Metabola: complete metamorphosis
3.2. Metabola: complete metamorphosis
 3.3. Gallinsecta: an illusion of convergence
3.3. Gallinsecta: an illusion of convergence
 References
References
|
Cladoendesis is a method of phylogeny analysis by means of creating a
classification, in which each phylogenetic branch is represented by a
holophyletic taxon, each non-resolved area of phylogenetic tree is represented
by a plesiomorphon, and all taxa (holophyletic and plesiomorphons) are supplied
with own names; diagnosis of each taxon is supplied with references to
diagnoses of higher taxa, so that characters
of all taxa are formulated as hiearachically connected ones. |
Abstract—Cladoendesis is a method of phylogeny analysis opposed to various matrix methods. In contrast to matrix methods, in cladoendesis phylogenetic trees are not built each time as new ones, but are reconstructed based on the previous results. Each character of each taxon is compared with its ancestral condition in the ground-plan of the higher taxon. The revealed part of the phylogeny is represented in a form of hierarchical classification. In addition to the principles of natural classification elaborated by C. Linnaeus, P.-A. Latreille, and others,
evolutinory theory elaborated by Ch. Darwin and others,, and principles of phylogenetic systematics elaborated by W. Hennig and others, cladoendesis includes rank-free dual nomenclature system (DualNom) and advanced method of text layout elaborated by the author. Important components of cladoendesis are the use of the term “plesiomorphon” and taking into account such important evolutionary events as conservation and deconservation of characters. Cladoendesis allowed the author to make comprehensive revision of the phylogeny of Ephemeroptera, find homology in the structure of the maxillae of adult Aphaniptera, larval maxillae of Neuropteroidea-Birostrata, larval legs of Nannomecoptera, and genital parts of some insect taxa. It also allows understanding the nature and evolution of insect metamorphosis. It is generally believed that initially insect ontogenesis proceeds the shortest way, while such phenomena as substitutions of organs by means of their atrophy and subsequent restoring are secondary. In contrast to this, the flagellum of the antenna of Amyocerata initially grows by addition of proximal segments and simultaneous loss of distal ones; in many taxa, including Metabola (insects with complete metamorphosis), distal segments are not aborted. Some authors tried to explain the origin of complete metamorphosis by various reasons: ecological, morphogenetic, or others. This approach is wrong because all insects with complete metamorphosis constitute the holophyletic taxon Metabola Burmeister 1832, i.e., originate from a single ancestral species which acquired this kind of metamorphosis. If complete metamorphosis could appear in response to some factor, it would appear many times in different species, in which case the taxon Metabola, characterized by complete metamorphosis, would be polyphyletic. The holophyly of Metabola is well proven by cladoendesis but cannot be revealed by any matrix method. Based on understanding of these facts, the author was able to discover the specific features which appeared in the common ancestor of Metabola and then became conserved and were inherited by all its descendants. These features include loss of the scape in the larval antenna (leading to a peculiar transformation of antennae in metamorphosis) and a peculiar mode of leg transformation during molt from larva to pupa. During the larval/pupal molt, the leg loses musculature and gets an immobile knee bend, so that the pupa is unable to use its legs. This conserved feature determines the inactive mode of life of the pupae of most insects. It is usually believed that male coccids (Gallinsecta De Geer 1776) have metamorphosis similar to the complete metamorphosis of Metabola. But the phylogenetic position of Gallinsecta and Metabola, as revealed by cladoendesis, does not allow one to assume common modifications in their metamorphoses. Now, when concrete autapomorphies of Metabola have been found, it becomes possible to compare metamorphoses of Metabola and Gallinsecta. Examination of
Orthezia urticae and some other coccids shows that metamorphosis of their males shares no modifications with the true complete metamorphosis. The inactive mode of life of their nymphs is not connected with any anatomical reason, but purely with the fact that in the course of transformation from the feeding wingless larva to the non-feeding winged adult, the nymph has already lost its mouth apparatus and has not yet got functional wings, so it does not need to move. At the same time, actively feeding stages of Gallinsecta, both males and females, have an unusual mode of molt transformation of the legs and antennae; this is a unique autapomorphy of Gallinsecta not found in any other insect taxon.
Introduction.
In the modern literature, the names “phylogenetic analysis” or “cladistic analysis” are often applied to
procedures that clearly have nothing to do with phylogeny (for example, analysis of intraspecific forms)
or are inconsistent with scientific methodology (for example, analysis of phylogeny based on the “parsimonious evolution” scenario, which is not supported
by the existing theories).
In the recent decade, an efficient method of phylogenetic analysis
(cladoendesis) was developed by the author; this method represents extension and improvement of the traditional phylogenetic studies by building the natural classification.
The principles of cladoendesis were initially developed for the purpose of studying the phylogeny of mayflies (Ephemeroptera)
(Kluge 2004a); they allowed the author to understand better the homologies in the genital morphology of insects
(Kluge 2003 ), to find a mistake in the description of the larval legs of scorpionflies (Mecoptera)
(Kluge 2004b
), to find a mistake in the description of the larval legs of scorpionflies (Mecoptera)
(Kluge 2004b ), and to improve the understanding of the nature of
metamorphosis in different insect taxa and transformation of different appendages during molts
(Kluge 2005a
), and to improve the understanding of the nature of
metamorphosis in different insect taxa and transformation of different appendages during molts
(Kluge 2005a ,
2010d
,
2010d ). In particular, the study of molt-related transformations allowed the author to find homologies of parts of the imaginal maxilla in Aphaniptera
(Kluge 2002
). In particular, the study of molt-related transformations allowed the author to find homologies of parts of the imaginal maxilla in Aphaniptera
(Kluge 2002 ) and the larval maxilla in Neuropteroidea-Birostrata
(Kluge 2005b
) and the larval maxilla in Neuropteroidea-Birostrata
(Kluge 2005b ).
).
In this paper, I use the principles of cladoendesis to consider the evolution of ontogenetic transformations of the insect legs and antennae.
1. THE PRINCIPLES OF CLADOENDESIS
The term “cladoendesis” was introduced recently (Kluge
& Novikova 2011) to designate the new approach to phylogenetic reconstructions which had been developed by the author for over a decade. The term
"cladoendesis" (literally meaning “branch coupling,” from the Greek words κλαδος + εν-δεσις) refers to the fact that in this approach, attention is focused on the connection between apomorphies of each taxon and characteristics of higher taxa, so that the characters of all the taxa are from the very beginning considered to be interrelated within a certain hierarchy.
In this respect, cladoendesis can be contrasted with the widely used matrix-based methods of phylogenetic reconstruction in which all the characters are treated as completely self-contained and are initially entered in a rectangular matrix rather than in a hierarchical system. In the matrix methods, phylogeny is reconstructed from scratch every time, as if it has never been reconstructed before. Such an approach would make sense if we had methods of phylogenetic reconstruction capable of producing the definitive and unambiguous result (as, for example, in chemical analysis). However, since in the process of phylogenetic reconstruction we seek to reveal the evolutionary events that have occurred only once in the past, no method of analysis can guarantee a perfectly correct result. Therefore, applying different methods of reconstruction and comparing the phylogenies obtained makes no sense. The only way to reconstruct phylogeny is to make use of all the available facts and theories in order to build a single model and to try to make this model as close to the actual phylogeny as possible. It should be borne in mind that it is basically impossible to obtain a phylogenetic model with a predefined degree of accuracy. Thus, even if we choose to overlook the evidently non-scientific nature of such a popular matrix method as parsimony analysis, we have to conclude that any matrix-based method has no prospects.
Cladoendesis is a direct descendant of that traditional method of phylogenetic analysis which far predated the theory of evolution and which actually served as the basis of the theory of evolution and the concept of phylogeny. The main milestones in the development of cladoendesis were the works of Aristotle (who recognized the hierarchical pattern in the diversity of animals), C. Linnaeus (who developed an efficient method to describe this hierarchy), G. Cuvier, J.-B. Lamarck, P.-A. Latreille, and other authors of the French Revolution epoch (who posed the question about the nature of this pattern), and Ch. Darwin (who explained the mechanism of evolution and thus established the evolutionary approach in biology). These authors and their successors were convinced that the hierarchical pattern in the diversity of animals reflected some real relationships, and that the nature of this pattern could be understood by describing it
in a form of hierarchical classification. Ch. Darwin clearly expressed the idea that the hierarchical classification of living organisms which had been developed since C. Linnaeus reflected their genealogy (i.e., phylogeny in E. Haeckel’ terminology):
“So that we here have many species descendent from a single progenitor grouped into genera; and the genera in sub-families, families, and orders, all in one great class. Thus, the grand fact of the natural subordination of all organic beings in groups under groups … is in my judgment explained.”
(Darwin 1871: 373 ). However, after the works of Ch. Darwin some biologists started to think that classification (which predated the
discovery of phylogeny) does not reflect phylogeny but instead represents something
else (although none of them could explain what else). In fact, however, “no other explanation
has ever been at-tempted” (Darwin 1871: 373
). However, after the works of Ch. Darwin some biologists started to think that classification (which predated the
discovery of phylogeny) does not reflect phylogeny but instead represents something
else (although none of them could explain what else). In fact, however, “no other explanation
has ever been at-tempted” (Darwin 1871: 373 ). Therefore, an important step in the development of cladoendesis were the works of W. Hennig who introduced the concept of paraphyly and clearly formulated the principles to which any classification meant to reflect phylogeny should adhere
(Hennig 1950).
). Therefore, an important step in the development of cladoendesis were the works of W. Hennig who introduced the concept of paraphyly and clearly formulated the principles to which any classification meant to reflect phylogeny should adhere
(Hennig 1950).
The main principle of cladoendesis is that all the data are entered in a classification in which all the characters are attributed to hierarchically subordinated taxa. The new data are added to the already existing classification, which is expanded and improved in the process, or corrected if errors are revealed. In order to perform this function, a classification should be able to accommodate all the newly obtained facts and to allow them to be easily found. The principles of classification developed by Linnaeus (1758) and Latreille (1802) contributed much to solving this problem: owing to these principles, data accumulation reached such a level which allowed the theory of evolution to be created and the presently known part of the phylogeny to be reconstructed. The issue that has remained unsolved until recently is related to the fact that all the principles of systematics and nomenclature considered classification within the preset system of absolute artificial ranks, because of which the data on all the hierarchically subordinated phylogenetic branches could not be entered in a convenient way.
To solve this problem, I have developed a rank-free nomenclature or, to be more precise, a
dual nomenclature system (DualNom) which allows an unlimited number of taxa distributed over an unlimited number of hierarchical levels to be given monosemantic names that do not contradict either the International Code of Zoological Nomenclature or the traditional usage of higher-taxa names
(Kluge 1999a, 1999b , 2000,
2009a, 2010с). This nomenclature was successfully used in practice: in particular, during full-scale revision of the
phylogenetic system of Ephemeroptera
(Kluge 2004a, 2007a, 2007b, 2008, 2009, 2010a, 2010b; Kluge & Novikova 2011). In this respect, the new system essentially differs from the PhyloCode and some other speculative nomenclature systems which have
no practical use (Kluge 1910c).
, 2000,
2009a, 2010с). This nomenclature was successfully used in practice: in particular, during full-scale revision of the
phylogenetic system of Ephemeroptera
(Kluge 2004a, 2007a, 2007b, 2008, 2009, 2010a, 2010b; Kluge & Novikova 2011). In this respect, the new system essentially differs from the PhyloCode and some other speculative nomenclature systems which have
no practical use (Kluge 1910c).
Another new feature of cladoendesis, besides the rational nomenclature, is the rational presentation of material. Instead of being repeated in different sections, such as diagnosis, description, comparative remarks, etc., each character is described only once, in the characteristic of the taxon whose autapomorphy this character represents. The description of a character contains references to the characters of higher-ranked taxa pertaining to the same morphological element; thus, autapomorphies of each taxon are explicitly compared with the primitive variant for a higher rank.
Proceeding from the assumption that every character showing evolutionary conservatism (see 2) and having taxonomic significance is an autapomorphy of a certain holophyletic taxon, one can reveal new holophyletic taxa, thus improving the phylogenetic reconstruction.
If classification and nomenclature of taxa are limited by a preset system of ranks, creation of new holophyletic taxa may prove impossible; on the contrary, rank-free nomenclature allows classification to be used for phylogenetic reconstruction without any limitation.
Of great importance for cladoendesis was the introduction of the term “plesiomorphon”
(Kluge 2004a: 15 ). A plesiomorphon is a taxon characterized only by plesiomorphies, so that there is no reason to consider such a taxon holophyletic; on the other hand, paraphyly of this taxon may not be proved either. To prove the paraphyly of a taxon, one should find a synapomorphy between part of this taxon and some other taxon. If such a synapomorphy is found, the former taxon should be regarded as paraphyletic and may be destroyed; part of it will be united with the latter taxon based on the apomorphy revealed, which in this case will become the autapomorphy of the new taxon. Thus, a phylogenetic classification does not contain any known paraphyletic taxa: a taxon is destroyed once its paraphyly has been demonstrated. However, plesiomorphons are necessarily present in any classification: they are merely assumed to be paraphyletic but in the absence of direct proof of paraphyly (i.e., the absence of knowledge of the corresponding apomorphies) they cannot be destroyed. Holophyletic taxa should be clearly distinguished from plesiomorphons. For this purpose, the name of a plesiomorphon should be preceded by the word “plesiomorphon” or by its abbreviated form “pm.” (in tables and indices), for example, “plesiomorphon Permoplectoptera” or “pm.Permoplectoptera.” The use of a similar term “plesion” instead of “plesiomorphon” is a mistake
(Kluge 2004a).
). A plesiomorphon is a taxon characterized only by plesiomorphies, so that there is no reason to consider such a taxon holophyletic; on the other hand, paraphyly of this taxon may not be proved either. To prove the paraphyly of a taxon, one should find a synapomorphy between part of this taxon and some other taxon. If such a synapomorphy is found, the former taxon should be regarded as paraphyletic and may be destroyed; part of it will be united with the latter taxon based on the apomorphy revealed, which in this case will become the autapomorphy of the new taxon. Thus, a phylogenetic classification does not contain any known paraphyletic taxa: a taxon is destroyed once its paraphyly has been demonstrated. However, plesiomorphons are necessarily present in any classification: they are merely assumed to be paraphyletic but in the absence of direct proof of paraphyly (i.e., the absence of knowledge of the corresponding apomorphies) they cannot be destroyed. Holophyletic taxa should be clearly distinguished from plesiomorphons. For this purpose, the name of a plesiomorphon should be preceded by the word “plesiomorphon” or by its abbreviated form “pm.” (in tables and indices), for example, “plesiomorphon Permoplectoptera” or “pm.Permoplectoptera.” The use of a similar term “plesion” instead of “plesiomorphon” is a mistake
(Kluge 2004a).
2. CONSERVATION OF CHARACTERS
Before discussing the evolution of metamorphosis, one should consider an important and still unexplained evolutionary mechanism, namely the conservation of characters. In the course of evolution, characters not only appear and disappear; they can also be conserved and deconserved. Conservation of a character is manifested in the loss of capacity for its further evolutionary changes. If this character happens to be harmful, evolution cannot obliterate it but can in some way eliminate its negative effect on survival. Therefore the stability of such a character cannot be explained by natural selection; instead, it must be based on some genetic mechanism. Character conservation cannot be studied by methods of experimental genetics because, once a character has been conserved, it can no longer be changed in an experiment. The phenomenon of character conservation is well known; traditional taxonomists specially look for conserved characters and use them as diagnostic ones. Most of the apomorphies underlying our phylogenetic concepts are conserved characters: unlike the non-conserved ones, they can be traced in the entire holophyletic taxon, providing proof of its holophyly.
It is generally believed that some categories of characters are more conservative and others are less conservative: for example, morphological characters are supposedly more conservative than behavioral ones; musculature is supposedly more conservative than skeleton, and so on. Some taxonomists try to convince themselves that once a character has proved to be taxonomically significant in one taxon, then a similar character found in another taxon will have the same taxonomic weight. Although this would be very convenient for practical taxonomy, in reality this is not so. In fact, using the words of C. Linnaeus,
“what is essential for establishing one genus, may be of no significance at all in another genus” (“Quae in uno genere ad Genus stabiliendum valent, minime idem in altero necessario praestant”)
(Linnaeus 1751 : 114, § 169). Each conserved character is a particular character of a particular taxon, and its conservation is a single evolutionary event. It is particular characters of particular taxa, rather than sets of analogous characters, that may be conserved and taxonomically significant; therefore, taxonomic characters cannot be adequately entered in the taxon/character matrix. In view of this, all the matrix-based methods of phylogenetic analysis are meaningless.
The concept of conserved characters plays a key role in cladoendesis, since the traditional way of building a natural classification is mostly based on finding conserved characters among others.
A good example of a conserved character is the presence of six legs in Hexapoda, or, more precisely, the presence of three thoracic segments, each capable of bearing one pair of legs. Given the enormous diversity of insects, not all of them need exactly three pairs of legs. If some legs happen to be unnecessary, they either remain non-functional (for example, fore legs in Nymphalidae) or disappear; if three pairs of legs are not enough, additional locomotor organs appear (for example, prolegs). However, the total number of segments differentiated as thoracic ones is always three. In experiments with fruit flies, the morphology of some thoracic segments can be altered (for example, in the bithorax mutation, the metathorax becomes similar to the mesothorax and bears an identical extra pair of wings) but the total number of thoracic segments cannot be changed. In other arthropods, however, the same character (the number of segments in the thorax or a similar tagma) may be not so strictly conserved. For example, most sea spiders (Pantopoda) possess four segments with walking legs but in some species this number is increased to five or six. In Eucrustacea the number of thoracic segments varies between the taxa, and in some cases (for example, in Notostraca and some Anostraca) it is even subject to individual variation.
The concept of character conservation is very important for understanding the evolution of metamorphosis.
3. CLADOENDESIS AND THE STUDY OF METAMORPHOSIS
The evolution of insect metamorphosis is often considered without any reference to phylogeny; some authors proposed classifications of types of metamorphosis which did not correspond to the phylogenetic classification of insects. In fact, however, each type of metamorphosis has appeared only once and is characteristic of a single phylogenetic lineage.
We consider the following phylogenetic classification of the taxa discussed here
 :
:
1. Hexapoda Blainville 1816 (nom.hier.: Scarabaeus/fg3)
1.1. Entognatha Stummer-Traunfels 1891 (nom.hier.: Podura/fg1)
1.2. Amyocerata Remington 1955 (nom.hier.: Scarabaeus/fg4)
1.2.1. Triplura Ewing 1942 (nom.hier.: Lepisma/fg1)
1.2.2. Pterygota Gegenbaur1878 (nom.hier.: Scarabaeus/fg5)
1.2.2.1. Ephemeroptera Hyatt & Arms 189 (nom.hier.: Ephemera/fg1)
1.2.2.2. Metapterygota Börner 1909 (nom.hier.: Scarabaeus/fg6)
1.2.2.2.1. Odonata Fabricius 1793 (nom.hier.: Libellula/fg1)
1.2.2.2.2. Neoptera Martynov 1923 (nom.hier.: Scarabaeus/fg7)
1.2.2.2.2.1. Plesiomorphon Polyneoptera Martynov 1923 (nom.hier.: Gryllus/f1=Forficula/g1)
1.2.2.2.2.2. Eumetabola Hennig 1953 (nom.hier.: Scarabaeus/fg8)
1.2.2.2.2.2.1. Metabola Burmeister 1832 (nom.hier.: Scarabaeus/fg9)
1.2.2.2.2.2.2. Parametabola Crampton 1938 (nom.hier.: Cimex/f1=Cicada/g1)
1.2.2.2.2.2.2.1. Zoraptera Silvestri 1913 (nom.hier.: Zorotypus/fg1)
1.2.2.2.2.2.2.2. Acercaria Börner 1904 (nom.hier.: Cimex/f2=Cicada/g2)
1.2.2.2.2.2.2.2.1. Panpsocoptera Crampton 1938 (nom.hier.: Psocus/f1=Pediculus/g1)
1.2.2.2.2.2.2.2.2. Condylognatha Börner 1904 (nom.hier.: Cimex/f3=Cicada/g3)
1.2.2.2.2.2.2.2.2.1. Thysanoptera Haliday 1836 (nom.hier.: Thrips/fg1)
1.2.2.2.2.2.2.2.2.2. Arthroidignatha Spinola 1850 (nom.hier.: Cimex/f4=Cicada/g4)
1.2.2.2.2.2.2.2.2.2.1. Hemelytrata Fallen 1829 (nom.hier.: Cimex/f5=Cicada/g5)
1.2.2.2.2.2.2.2.2.2.2. Plantisuga Dumeril 1806 (nom.hier.: Aphis/fg1)
1.2.2.2.2.2.2.2.2.2.2.1. Psyllaleyroda Kluge 2010 (nom.hier.: Psylla/fg1)
1.2.2.2.2.2.2.2.2.2.2.1.1. Saltipedes Amyot & Serville 1843 (nom.hier.:
Psylla/fg2)
1.2.2.2.2.2.2.2.2.2.2.1.2. Scytinelytra Amyot & Serville 1843 (nom.hier.:
Aleyrodes/fg1)
1.2.2.2.2.2.2.2.2.2.2.2. Aphidococca Kluge 2010 (nom.hier.: Aphis/fg2)
1.2.2.2.2.2.2.2.2.2.2.2.1. Gynaptera Laporte 1834 (nom.hier.: Aphis/fg3)
1.2.2.2.2.2.2.2.2.2.2.2.2. Gallinsecta De Geer 1776 (nom.hier.: Coccus/fg1)
The abbreviation “nom. hier.” designates here the typified rank-free hierarchical name (nomen hierarchicum) of the same taxon for which the non-typified rank-free circumscriptional name (nomen circumscribens) is provided. The statuses of the names used in this classification were discussed earlier (Kluge, 2010c, 2010d).
For the purpose of adequate description of metamorphosis, some new Latin terms had to be introduced
(Kluge 2010d ) in addition to such common terms as “first instar larva,” “second instar larva,” “second instar nymph,” etc. and the corresponding abbreviations “L1,” “L2,” “N2.” Each term is a single compound word:
primolarva, secundolarva, secundonympha, etc. The processes of transformation during molt from one instar to another can be described using adjectives derived from these terms: primolarval, secundolarval, etc. Since the terms “larva” and “nymph” do not have universally accepted definitions, the instars should always be named with continuous numbering: for example, if the first two instars are commonly referred to as larval, and
others – as nymphal, then the secundolarva will be followed by the tertionympha (and not by the primonympha). Since the last two instars tend to be more stable, they can be designated by the terms
ultimonympha and penultimonympha, or the parallel terms ultimolarva and
penultimolarva. To maintain uniform and continuous numbering, the last instar larva of insects with complete metamorphosis should be called “penultimolarva”; the terms “ultimolarva” and “ultimonympha” should not be used in this case because the last preimaginal instar is commonly referred to as pupa.
) in addition to such common terms as “first instar larva,” “second instar larva,” “second instar nymph,” etc. and the corresponding abbreviations “L1,” “L2,” “N2.” Each term is a single compound word:
primolarva, secundolarva, secundonympha, etc. The processes of transformation during molt from one instar to another can be described using adjectives derived from these terms: primolarval, secundolarval, etc. Since the terms “larva” and “nymph” do not have universally accepted definitions, the instars should always be named with continuous numbering: for example, if the first two instars are commonly referred to as larval, and
others – as nymphal, then the secundolarva will be followed by the tertionympha (and not by the primonympha). Since the last two instars tend to be more stable, they can be designated by the terms
ultimonympha and penultimonympha, or the parallel terms ultimolarva and
penultimolarva. To maintain uniform and continuous numbering, the last instar larva of insects with complete metamorphosis should be called “penultimolarva”; the terms “ultimolarva” and “ultimonympha” should not be used in this case because the last preimaginal instar is commonly referred to as pupa.
When describing the complete metamorphosis in insects, some authors stated that it included a repeated organogenesis of appendages or even a repeated embryogenesis. The imaginal discs of Cyclorrhapha are sometimes described as some “embryonic” tissues concealed with the larval body, which undergo a kind of de novo embryogenesis to form the adult fly. The concept of cladoendesis has no place for such fantasy since it requires that metamorphosis of any organism should be considered with reference to the already established phylogenetic position of the corresponding taxon. The higher flies (Cyclorrhapha) belong to
Hexapoda Blainville 1816, which belong to
Arthropoda Siebold 1848, which, in turn, belong to
Monostomata Huxley 1875 (= Eumetazoa Butschli 1910). The taxon Monostomata is characterized, among other features, by irreversible differentiation of the blastoderm into the ectoderm and the endoderm-mesoderm primordium that takes place at early stages of embryogenesis. The Arthropoda are characterized by a single anlage of appendages on each segment, while the Hexapoda are characterized by the unique and highly conservative bauplan of thoracic appendages (legs), which is not found in other arthropods. At the same time, all the Arthropoda possess the ability for regeneration of appendages: if part of an appendage is amputated, the remaining part develops into the normal appendage with a complete set of segments (for example, in Hexapoda the remaining coxa and trochanter can develop into the leg comprising the coxa, trochanter, femur, tibia, and tarsus). Thus, during regeneration the ontogenetic homology of segments is disrupted but the ontogenetic homology of the appendage as a whole is preserved (for explanation of the term “ontogenetic homology,” see
Kluge 2005a ). The same abilities are realized during normal development in any case of modified metamorphosis: the new appendage does not appear anew and does not develop from some enigmatic “imaginal disc”; instead, it develops from the remaining tissues of the preceding appendage, whereas its segmentation may indeed appear anew. Even such intricate structures as the imaginal discs of Cyclorrhapha are merely modified pre-molt integumental folds typical of all the arthropods
(Kluge 2005a).
). The same abilities are realized during normal development in any case of modified metamorphosis: the new appendage does not appear anew and does not develop from some enigmatic “imaginal disc”; instead, it develops from the remaining tissues of the preceding appendage, whereas its segmentation may indeed appear anew. Even such intricate structures as the imaginal discs of Cyclorrhapha are merely modified pre-molt integumental folds typical of all the arthropods
(Kluge 2005a).
3.1. Amyocerata: the evolution of molt-related processes in the antenna
It is generally believed that in the most primitive metamorphosis, development follows the shortest way, whereas such phenomena as disappearance and subsequent restoration of an organ have a secondary nature. This, however, is not always so.
The taxon Amyocerata Remington 1955 is often erroneously referred to as Ectognatha or Insecta, which leads to confusion
(Kluge 1996, 1999b, 2000, 2010c). In reality Amyocerata is a holophyletic taxon within the holophyletic taxon Hexapoda. The most vivid autapomorphy of Amyocerata is the structure of their antennae: the antenna initially consists of a one-segmented scape, a one-segmented pedicel, and a
multisegmented flagellum; muscles extend from the tentorium or some other skeletal element inside the head to the base of the scape; the scape contains muscles extending from its base to the base of the pedicel; there are no other muscles in the antenna; evolution of the antennae in Amyocerata may include reduction of muscles but never includes development of new segments with muscles. It should be noted that these characters are highly conservative: regardless of the size, shape, and function of the antennae in Amyocerata, they never have muscles in any segment except the scape; at the same time, the muscles of the scape are preserved even in strongly shortened antennae and are reduced only in extremely rare cases. No exceptions have been found since this character had been formulated by Imms
(1939 ).
).
During each molt in the course of ontogenesis, the scape (with its muscles) and pedicel are preserved, changing only in size and/or shape; the number of segments in the flagellum usually remains the same or increases. The number of flagellomeres increases due to growth and division of the proximalmost segment; one or several additional growth zones may be present in other parts of the flagellum as well (Imms
1940 ). It is interesting that in some cases, the addition of segments in the proximal part of the flagellum is accompanied by necrosis and detachment of several distal segments; although the total length of the flagellum and the number of its segments usually increase. The remaining tissues of the dead flagellomeres are not completely resorbed but are shed together with the exuvia, so that this type of molt can be revealed by examination of exuviae (by contrast with similar processes in the legs of some insects, when all the remains are resorbed). In the antenna molting in this way, the apical flagellomere has no specific morphological features, because during each molt one of the middle segments becomes the apical one. In some cases, the shedding of the distal portion of the flagellum may be explained by purely mechanical reasons: the apical portion of the flagellum may be so thin that it gets torn off during ecdysis and remains within the old cuticle. In some cases, however, the tissues are severed at such a level where the thickness of the flagellum would not hinder its molt, and not during ecdysis but long before it (Kluge, 2010d: fig.
24
). It is interesting that in some cases, the addition of segments in the proximal part of the flagellum is accompanied by necrosis and detachment of several distal segments; although the total length of the flagellum and the number of its segments usually increase. The remaining tissues of the dead flagellomeres are not completely resorbed but are shed together with the exuvia, so that this type of molt can be revealed by examination of exuviae (by contrast with similar processes in the legs of some insects, when all the remains are resorbed). In the antenna molting in this way, the apical flagellomere has no specific morphological features, because during each molt one of the middle segments becomes the apical one. In some cases, the shedding of the distal portion of the flagellum may be explained by purely mechanical reasons: the apical portion of the flagellum may be so thin that it gets torn off during ecdysis and remains within the old cuticle. In some cases, however, the tissues are severed at such a level where the thickness of the flagellum would not hinder its molt, and not during ecdysis but long before it (Kluge, 2010d: fig.
24 ). Molts with such a predetermined loss of the distal portion of the flagellum are known in Triplura (both in Zygentoma and in Microcoryphia), Ephemeroptera, and Plecoptera. Judging by the systematic position of these taxa, this paradoxical way of flagellum growth is primitive for Amyocerata.
). Molts with such a predetermined loss of the distal portion of the flagellum are known in Triplura (both in Zygentoma and in Microcoryphia), Ephemeroptera, and Plecoptera. Judging by the systematic position of these taxa, this paradoxical way of flagellum growth is primitive for Amyocerata.
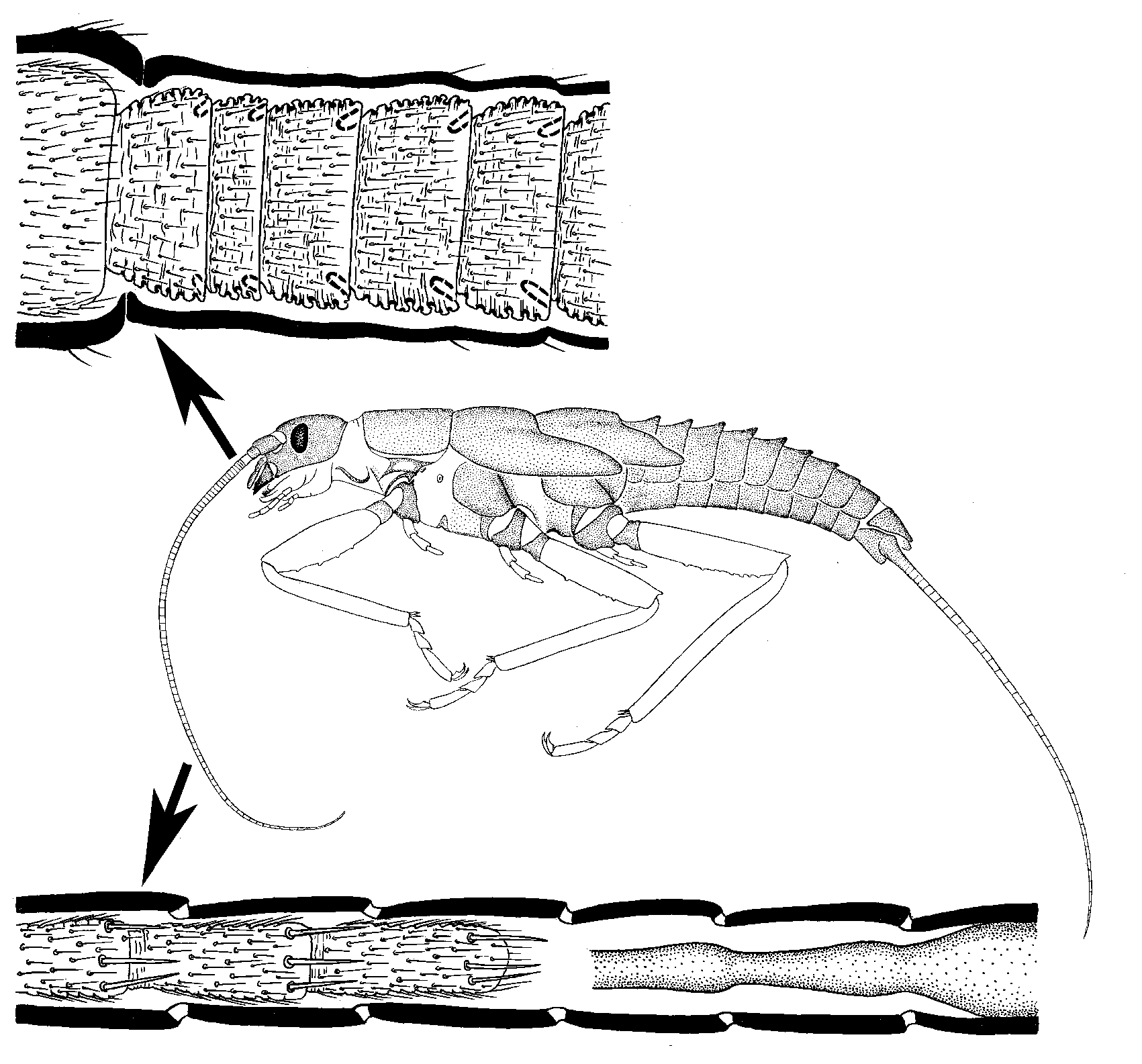
A similar process of addition of proximal segments and loss of distal ones is observed in the multisegmented
caudalii of Triplura, Ephemeroptera, and Plecoptera.
In mayflies (Ephemeroptera), the multisegmented flagellum of the larva is transformed into a thin non-segmented annulate flagellum of the
subimago during the penultimate molt. As in the preceding larval molts, the distal portion of the living tissues of the flagellum is lost and remains in the larval exuvia. This happens regardless of whether or not the total length of the flagellum decreases.
In Odonata, many Polyneoptera (Embioptera, Isoptera, Dermatoptera, Spectra, etc.), and all the Parametabola the antennal flagellum has a permanent apex which is not shed during molts. To all appearances, this mode of development was acquired independently in different phylogenetic lineages. In Cercopoidea (which belong to Parametabola) the last molt is accompanied by a process externally resembling the larval-subimaginal transformation of the flagellum in mayflies: the 7-segmented flagellum of the larva is transformed into the non-segmented one of the adult; however, contrary to the situation in mayflies, no tissues are lost. In the taxon Gallinsecta (also belonging to Parametabola) the entire antenna undergoes an unusual transformation during which the distal portion of the flagellum is necrotized and detached (see 3.3); in spite of this, the antenna may possess some apical structures peculiar for the ultimate flagellomere (Kluge,
2010d ).
).
In all insects with complete metamorphosis (Metabola) the larval antenna has lost its original morphology typical of Amyocerata (see 3.2). Despite the extensive transformations taking place during molts from the larva to the pupa and then to the adult, no antennal tissues are necrotized or detached. It is generally assumed that complete metamorphosis differs from other types in more profound necrotic processes; however, the situation with the distal portion of the antenna is exactly opposite: the initial mode of antenna development in Amyocerata involves necrosis whereas in Metabola this feature has been lost.
3.2. Metabola: complete metamorphosis
Although complete metamorphosis is a well known phenomenon and the relevant literature is extensive, until recently it was not clear what exactly constituted complete metamorphosis. Externally, this type of development is manifested in the presence of the following stages: (1) a motile feeding larva resembling a worm; (2) an inactive pupa, completely immobile or capable of only limited movements; and (3) an adult. It has remained unknown why the larvae (despite their highly variable morphology) are commonly associated with worms and what exactly limits the mobility of all the pupae.
Already by the beginning of the XX century it has become commonly accepted that all the insects with complete metamorphosis form a holophyletic taxon
Metabola Burmeister 1832; the names
Holometabola Burmeister 1835 and Endopterygota
Sharp 1899 are often used to designate this taxon but this is a mistake since these names were originally proposed for taxa with a different composition. The name
Oligoneoptera Martynov 1923, often used in the modern literature, is a junior circumscriptional synonym (Kluge, 2000,
2010c ) of the name Metabola.
) of the name Metabola.
The proofs of holophyly of Metabola may be formulated as follows: in any taxon reliably shown to be holophyletic based on unquestionable autapomorphies,
(1) either all the species have complete metamorphosis, with no exceptions; (2) or all the species have a different type of metamorphosis, with no exceptions;
(3) or (in case of a larger taxon including Metabola) the taxon includes all the species with complete metamorphosis.
The validity of this statement cannot be tested directly because not all insect species are presently known, and most of those scientifically described are known from several adult specimens only. In the XIX century the exact mode of metamorphosis was known for such a small number of species that the above statement could still be doubted. Now, however, the number of species whose development is known is so great that finding an exception appears totally improbable. At the present level of knowledge, we can assume that a new species of butterflies with unusual morphological characters may be found; but we cannot assume that a butterfly will ever be found that does not have complete metamorphosis. The holophyly of Metabola can be substantiated only by the principles of cladoendesis: it follows from the enormous experience of a great number of taxonomists and other researchers and observers, summarized in the vast taxonomic literature. Although the researchers did not know until recently what features constituted complete metamorphosis, they were perfectly able to recognize this type of metamorphosis and distinguish it from other types.
Many authors tried to explain the origin of complete metamorphosis by various reasons: ecological, morphogenetic, embryological, and others. However, if Metabola represent a holophyletic taxon, none of these explanations can be true: if there existed a certain cause that had once led to the appearance of complete metamorphosis, it would produce the same type of metamorphosis repeatedly, in which case the taxon uniting all the insects with complete metamorphosis would not be holophyletic. The holophyly of Metabola essentially means that complete metamorphosis appeared in one Late Paleozoic species which co-existed with many other pterygote insects; this evolutionary act was accidental and so unlikely that it has never been repeated since that time. Thus, one should ask not why complete metamorphosis appeared, but which specific traits were acquired by the common ancestor of Metabola and inherited by all its descendants. From this point of view, complete metamorphosis has been hardly considered at all. My earlier paper
(Kluge 2005a ) presented the first attempt to describe the specific autapomorphies of Metabola revealed by the study of only several representatives of this group.
) presented the first attempt to describe the specific autapomorphies of Metabola revealed by the study of only several representatives of this group.
The peculiar transformation of the legs during the molt from the larva to the
pupa proved to be the key feature of Metabola: the living leg tissues first degenerate, strongly diminishing in size and losing the muscles, and then grow in such a way that an immobile knee bend is formed; therefore, after shedding of the larval cuticle the legs of the pupa are always bent at the knees and have no muscles to extend
them. This particular trait was so well conserved that it could not be altered by any subsequent evolutionary changes. As a result, the pupae of all the Metabola had to adapt to the immobility of their legs in different ways: in most cases they switched to inactive, sheltered mode of life; in rarer cases (for example, in Culicomorpha) they acquired a new mode of locomotion which would not be hindered by immobile legs. It was thus explained why the pupae incapable of locomotion still perform useless body movements.
Having no original data on the metamorphosis of Hymenoptera Symphyta, in my earlier communication I considered their larvae to be “short-legged,” like those of Lepidoptera
(Kluge 2005a: 217 ), based on the paper of Ivanova-Kazas
& Ivanova (1964) who stated that “no degenerative changes in the leg hypoderm were observed during the process.” However, my later observations of several sawfly species (Tenthredinoidea:
Diprion pini L., Cimbex femorata L., and others) showed that their legs developed by the “longlegged” type, i.e., that the larval-pupal transformation involved not only a complete loss of the leg muscles but also a profound reduction of the hypoderm.
), based on the paper of Ivanova-Kazas
& Ivanova (1964) who stated that “no degenerative changes in the leg hypoderm were observed during the process.” However, my later observations of several sawfly species (Tenthredinoidea:
Diprion pini L., Cimbex femorata L., and others) showed that their legs developed by the “longlegged” type, i.e., that the larval-pupal transformation involved not only a complete loss of the leg muscles but also a profound reduction of the hypoderm.
The worm-like appearance of the larvae of most Metabola, although differently expressed in different groups, may be explained by the fact that the larva of the common ancestor of Metabola had very short legs with a complete set of segments and muscles typical of Hexapoda (among recent insects, such legs can be found in the larvae of many Lepidoptera); in locomotion this larva mostly depended on the movements of its vermiform body. Further evolution of the larvae proceeded in different directions: either to reduction and even a complete loss of the legs, or to their secondary elongation. Since the evolution of the larvae started from the vermiform type in all the cases, most of them still retain some traits of the original bauplan. The uniform growth pattern of the pupal leg (with invariable formation of the knee bend) and the amazing diversity of the ways of partial degeneration of the larval leg tissue preceding it suggest that shortening of the larval legs took place only once, in the common ancestor of Metabola, whereas secondary elongation of the larval legs occurred independently in different taxa of Metabola.
The new observations suggest that all the Coleoptera Polyphaga possess the same mode of larval leg degeneration, involving violation of the ontogenetic homology of segments, which was described in detail for the mealworm beetle
Tenebrio molitor L. and the Colorado potato beetle Leptinotarsa decemlineata Say
(Kluge 2005a ). A completely different mode of leg degeneration occurs in Coleoptera Adephaga. In the great diving beetle
Dytiscus marginalis L. and some ground beetles (Carabidae), tissues of the distal portion of the larval leg undergo necrosis and lysis but the hypoderm is not detached from the cuticle; the pupal leg then develops from the tissues remaining in the proximal part of the larval leg.
). A completely different mode of leg degeneration occurs in Coleoptera Adephaga. In the great diving beetle
Dytiscus marginalis L. and some ground beetles (Carabidae), tissues of the distal portion of the larval leg undergo necrosis and lysis but the hypoderm is not detached from the cuticle; the pupal leg then develops from the tissues remaining in the proximal part of the larval leg.
Besides the peculiar mode of leg transformation, the taxon Metabola is characterized by the unique development of the antenna. The larval antenna does not follow the amyocerate bauplan: it has no segment corresponding to the scape and no internal muscles; in case of a multisegmented larval antenna, none of its segments corresponds to any segment in the adult. During metamorphosis, the imaginal antenna develops from the entire larval antenna without necrosis of any part, but the larval segmentation is completely lost and replaced with the new, imaginal one. Thus, the antennae of the larva and the adult are homologous both ontogenetically and phylogenetically but the individual antennal segments reveal neither ontogenetic nor phylogenetic homology. The scape muscles appear only during the pupal development. Unlike the legs, which may become mobile at the end of the pupal stage in some Metabola, the antennae always remain immobile until the very imaginal molt.
Other autapomorphies of Metabola were described in the cited paper (Kluge 2005a ).
).
3.3. Gallinsecta: an illusion of convergence
It is commonly believed that metamorphosis of male coccids (scale insects—Gallinsecta De
Geer 1776) resembles the complete metamorphosis. Indeed, ontogenesis of the male coccid includes the stage of an actively feeding larva that has no protoptera and differs strongly from the adult; the stage of a non-feeding, immobile, sheltered nymph; and the stage of a winged adult that differs strongly from the larva. The similarity between the ultimonympha of Gallinsecta and the pupa of Metabola is enhanced by the fact that the ultimonympha has immobile antennae, directed backwards
(Kluge 2010d: fig. 4 ). The pupae of some Metabola (Trichoptera, Raphidioptera, Chrysopidae, etc.) become capable of extending their legs and walking on them immediately before molting to the adult (i.e., on the pharate adult phase) but their antennae still remain immobile and backward-directed
(Kluge 2005a: fig. 39
). The pupae of some Metabola (Trichoptera, Raphidioptera, Chrysopidae, etc.) become capable of extending their legs and walking on them immediately before molting to the adult (i.e., on the pharate adult phase) but their antennae still remain immobile and backward-directed
(Kluge 2005a: fig. 39 ).
).
Some authors even considered the coccid metamorphosis as an intermediate type between incomplete and complete metamorphosis, indicating that coccid females develop with incomplete, and males with complete metamorphosis. Most authors prefer a more roundabout way of expression, speaking of convergent similarity between the metamorphosis of male coccids and the complete metamorphosis of Metabola. However, the term “convergence” merely reflects the presence of similar traits whose appearance is not explained by the modern evolutionary theory.
The scale insects form the taxon Gallinsecta which is clearly holophyletic. As can be seen from the phylogenetic classification of insects provided above, Gallinsecta and Metabola cannot have any common characters save those present in other taxa of Eumetabola as well. In this classification, only the taxon Parametabola has a disputable status (since some authors disagree with the position of Zoraptera as a sister group of Acercaria). All the other taxa separating Metabola and Gallinsecta, namely Acercaria, Condylognatha, Arthroidignatha, Plantisuga, and Aphidococca, are unquestionably holophyletic. It is essential that holophyly of these taxa has been proved without any matrix-based methods of phylogenetic analysis. Each of these taxa is characterized by unique autapomorphies which are found nowhere else in the nature (i.e., the outgroup is not an arbitrarily chosen species but all the living organisms taken together), and the presence of these autapomorphies was verified by a great number of researchers for all the species known to them; no matrix would be able to accommodate such an amount of data.
In order to reveal the specific traits of similarity, the metamorphosis of the males of Gallinsecta had to be compared with the complete metamorphosis of Metabola. Until recently, this was an impossible task because the specific features of complete metamorphosis were unknown. Now, it has become clear that the key feature of complete metamorphosis is the peculiar mode of leg transformation, and that the immobility of the antennae in the pupae of Metabola results from the specific traits of their morphology in the larvae. Therefore, it is now possible to compare the ontogenetic processes in Metabola and Gallinsecta.
The nettle ensign scale Orthezia urticae L. was studied as a representative of Gallinsecta. The transformation of the legs and antennae during all the molts in both sexes of this species was described in detail in my earlier paper (Kluge,
2010d ). The research showed that the molts of non-feeding, “pupa-like” nymphs of males were not accompanied by any processes analogous to the transformation of legs and/or antennae during complete metamorphosis: there were no degenerating parts, all the muscles were preserved, each segment developed from the corresponding segment of the preceding instar and remained within its cuticle before the molt, so that all the segments preserved the potential for active movement in all the phases of the molting cycle.
). The research showed that the molts of non-feeding, “pupa-like” nymphs of males were not accompanied by any processes analogous to the transformation of legs and/or antennae during complete metamorphosis: there were no degenerating parts, all the muscles were preserved, each segment developed from the corresponding segment of the preceding instar and remained within its cuticle before the molt, so that all the segments preserved the potential for active movement in all the phases of the molting cycle.
The immobility and sheltered life of the penultimonympha and ultimonympha is probably explained by the fact that they simply do not need to move. The adult males of all the Gallinsecta can fly and mate but do not feed; therefore, during transformation from the larva to the adult they lose the mouthparts and acquire the wings and genitals. At the stages of
penultimonympha and ultimonympha, the mouthparts have already disappeared completely while the wings and genitals are present in the form of immobile and nonfunctional protoptera and
protopenis.
The same research showed the molts of the feeding stages (the first two instars of the male and all the instars of the female) to be accompanied by the unique transformation of all the appendages: legs and antennae. During each molt, the distal part of each appendage undergoes complete necrosis while the remaining proximal part loses its segmentation and all its internal muscles. The appendage then grows, surpassing its former size; its musculature and segmentation appear anew. During this process, the 1st segment of the appendage (the scape or the coxa) invaginates into the body in an inverted state, i.e., with integument on the outside and muscles on the inside. The proximal segments remain in this state until ecdysis, and are everted into the normal shape (with muscles on the inside) as the insect tries to get out of the broken old cuticle. The feeding stages of
O. urticae use their legs to keep on the host plant; despite such a drastic transformation of the legs, their attachment function is preserved during the entire molt cycle due to the fact that the muscles running from the body to the proximal margins of the coxa do not degenerate and do not change their insertion sites. As a result, after each molt all the segments of the legs and antennae become ontogenetically non-homologous to those of the corresponding appendages of the preceding instar. In O. urticae this process is not accompanied by any external morphological changes: all the consecutive instars give the appearance of gradual development, without any events of degeneration and replacement.
Judging by the fragmentary data available for several other species of Gallinsecta, one may suggest that the paradoxical development observed in
O. urticae is not the primitive type for Gallinsecta but a variant derived from a more complicated type of development, in which the motile 1st instar larva is followed by the legless 2nd instar larva, which is followed in turn by another motile stage with legs and antennae.
In any event, the metamorphosis of Gallinsecta is a unique autapomorphy of this taxon. Inversion of part of an appendage is not known in any other insect group, including whiteflies (Scytinelytra) or higher flies (Cyclorrhapha) with their highly unusual modifications of metamorphosis.
Tables 1 and 2 show simplified comparative schemes of metamorphoses in the males of Gallinsecta and in Metabola; the former taxon is represented by the nettle ensign scale
Orthezia urticae, and the latter, by the common green lacewing Chrysoperla carnea, because they have the same total number of instars. The scheme covering all the diverse variants of metamorphoses in Gallinsecta and Metabola would be much more complicated but it would reveal the same fact: the modified molts occur at different stages in Gallinsecta and in Metabola and also differ in the nature of modifications.
| Metabola: Birostrata |
L1 |
L2 |
L3 |
 |
P |
 |
imago |
Gallinsecta: Orthezia  |
L1 |
L2 |
N3 |
 |
N4 |
 |
imago |
Gallinsecta: Orthezia  |
L1 |
L2 |
L3 |
 |
L4 |
(imago) |
| WWWWWWWWWWW |
WWW |
WWW |
|
|
|
|
|
L = larva, N = nympha, P = pupa
|
WWWW |
antennae |
legs |
|
change
structure |
necrosis |
inversion |
change
structure |
necrosis |
inversion |
|

|
– |
– |
– |
– |
– |
– |
|

|
+ |
– |
– |
+ |
± |
– |
|

|
– |
+ |
+ |
– |
+ |
+ |
REFERENCES
Darwin Ch. 1871. On the origin of species by means of natural selection, or the preservation of favored races in the struggle for
life. 5th edition. New York: D. Appleton and company. 447 p. BHL
Hennig W. 1950. Grundzuge einer Theorie der phylogenetischen Systematik. // Deutscher
Zentralverlag, Berlin. 370 S.
Ivanova-Kazas O.M. & Ivanova N.A. 1964. Metamorphosis of the sawfly Pontania capreae L. (Hymenoptera,
Tenthredinidae). I. Hypoderm. // Entomol. Obozr. 43 (2): 309–326.
Imms A.D. 1939. On the antennal musculature in insects and other Arthropods. // Quarterly Journal of Microscopical
Science 81: 273–320. 
Imms A.D. 1940. On growth processes in the antennae of insects. // Quarterly Journal of Microscopical
Science 81: 585–593. 
Kluge N.J. 1996. Myths in insect systematics and principles of zoological
nomenclature. // Entomol. Obozr. 75 (4): 939–944. (in Russian) 
Kluge N.J. 1999a. A system of alternative nomenclatures of supra-species taxa. Linnaean and
post-Linnaean principles of systematics. // Entomol. Obozr. 78 (1): 224–243.
(in Russian)  [English translation: Entomol. Rev. 79 (2), 133–147].
[English translation: Entomol. Rev. 79 (2), 133–147].
Kluge N.J. 1999b. Mitos en sistematica y principos de nomenclatura zoologica. / Myths in systematics and principles of zoological
nomenclature. // Boletin de la Sociedad Entomologica
Aragonesa 26: 347–377. 
Kluge N.J. 2000. Modern systematics of insects. Part I. Principles of
systematics of living organisms and general system of insects, with
classification of primary wingless and paleopterous insects. // Lan, St.
Petersburg: 336 pp. [in Russian].
Kluge
N.J. 2002. The homology of mouthparts in fleas (Insecta, Aphaniptera). // Entomol. Obozr. 81
(4): 808–816 (in Russian)  [English translation: Entomol. Rev. 82 (8): 1020–1026].
[English translation: Entomol. Rev. 82 (8): 1020–1026].
Kluge
N.J. 2003. On Evolution and Homology of Genital Appendages in Insects,” Trudy Russ. Entomol. O-va 74, 3–16
(in Rusian). 
Kluge N.J. 2004a. The phylogenetic system of Ephemeroptera. // Kluwer Academic
Publishers. 442 p.
Kluge N.J. 2004b. Larval leg structure of Nannochorista and characteristics of Mecoptera. // Russian Entomological
Journal (2003) 12 (4): 349–354. 
Kluge N.J. 2005a. Larval/pupal leg transformation and a new diagnosis for the taxon Metabola
Burmeister, 1832 = Oligoneoptera Martynov, 1923. // Russian Entomological
Journal (2004) 13 (4): 189–229. 
Kluge N.J. 2005b. Metamorphosis and homology of mouthparts in Neuropteroidea (Hexapoda: Metabola), with remarks on systematics and nomenclature. // Russian Entomological
Journal 14 (2): 87–100. 
Kluge N.J. 2007a. Oligoneuria itayana sp.n. (Ephemeroptera, Oligoneuriidae) – a new mayfly species from Peruvian Amazonia. // Russian Entomological
Journal 16 (2): 127–137.
Kluge N.J. 2007b. Review of Ameletidae (Ephemeroptera) of Russia and adjacent lands. // Russian Entomological
Journal 16 (3): 245–258.
Kluge N.J. 2008. A new taxon Hermanellonota, or subtribe Hermanellini subtr.n. (Ephemeroptera, Leptophlebiidae, Hagenulini), with description of three new species from Peruvian Amazonia. // Russian Entomological
Journal (2007) 16 (4): 127–137.
Kluge N.J. 2009a. New version of the database “Ephemeroptera of the World” as the first experience of a permanent and objective web catalogue in biology. // Aquatic
Insects 31, supplement 1: 167–180. WWW
Kluge N.J. 2009b. Higher system of Atalophlebiinae (Leptophlebiidae) with description of three new species of
Terpides s.l. from Peruvian Amazonia. // Russian Entomological Journal
18 (4) 243–256.
Kluge N.J. 2010a. A new autapomorphy of the taxon Tricoryptera and redescription of
Ephemerythus. // Russian Entomological Journal 19 (1): 21–30.
Kluge N.J. 2010b. Redescription of the taxon Tricorygnatha (Ephemeroptera, Tricorythus s.l.) based on new findings in Africa and Indonesia. // Russian Entomological
Journal 19 (2): 79–104.
Kluge N.J. 2010c. Circumscriptional names of higher taxa in Hexapoda. //
Bionomina 1: 15–55. 
Kluge N.J. 2010d. Paradoxical molting process in Orthezia urticae and other coccids (Arthroidignatha, Gallinsecta). // Zoosystematica
Rossica 19 (2): 246–271. 
Kluge N.J. & Novikova E.A. 2011. Systematics of the mayfly taxon Acentrella (Ephemeroptera, Baetidae), with description of new Asian and African
species. // Russian Entomological
Journal 20 (1): 1–56.
Latreille P.A. 1802. Histoire naturelle, générale et particuliere des Crustacés et des
Insectes. T.3. Famille naturelle des genres. // Paris. BHL
Linnaeus C. 1751. Philosophia botanica. In qua explicantur fundamenta botanica cum definitionibus partium, exemplis terminorum, observationibus rariorum, adjectis figuris aeneis.
// Stockholmiae: apud Godofr. Kiesewetter, 1751. 362 p. BHL
// Русский перевод: К. Линней. Философия ботаники. Москава, "Наука"
1989: 452 с.
Linnaeus C. 1758. Systema Naturae per regna tria Naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. T.I.
// Holmiae: Impensis Direct. Laurentii Salvii: 824 p. BHL
![]()
![]()



